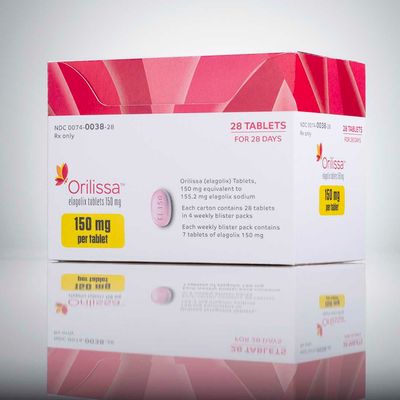
For the first time in ten years, a new drug will be on the market to help reduce pain caused by endometriosis. The drug company Abbvie announced that they received FDA approval for their drug Orilissa, which is intended to lessen suffering during the menstrual cycle and intercourse.
Endometriosis causes the uterine lining to grow outside of the uterus, which can lead to infertility, extreme cramping, and pain that feels like “tiny people skating on razor blades in your stomach.” Symptoms for endometriosis can often go unrecognized by doctors. Celebrities with the condition, like Lena Dunham and Padma Lakshmi, have brought increased awareness to the issue by openly discussing their own health complications.
According to the Star Tribune, the new treatment will cost $845 every four weeks without insurance. The drug works by reducing the production of estrogen. The pill reduced pain for about 75 percent of women given a higher dosage of the drug, and 45 percent given a lower dosage.
For people suffering with endometriosis, Orilissa will be a less costly and painful alternative to the pricey surgical procedures and side-effect heavy injections of hormone-suppressing drugs.